P3C
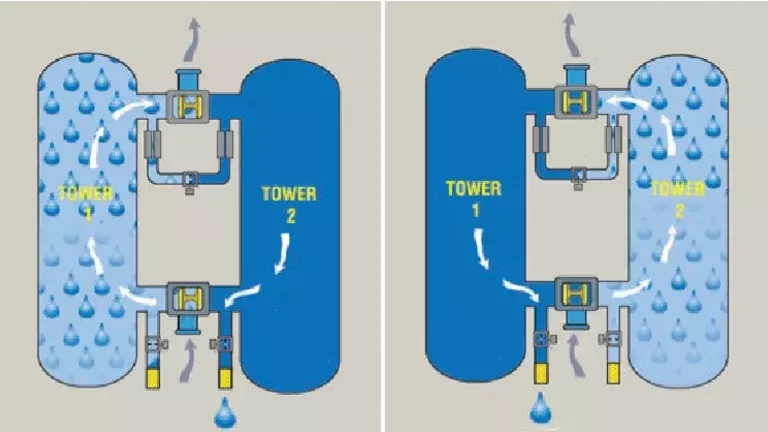
Dryer
Dual setting purge control with dryer purge activation.
or more information
When the objective is maintaining Medical air USP compliance and economizing purge air loss, our P3C (Patient Protect Purge Control) Dryer is the perfect solution.
P3C provides a dual setting purge control, with dryer purge activation based on achieving either a specific moisture or carbon dioxide trigger level. The result is guaranteed medical air USP acceptable levels for moisture and carbon dioxide and optimal purge economization.
#1
Measured breach of USP parameters is CO2 - The root cause is desiccant dryers!
The CO2 Conundrum
Dessicant dryers are commonly used to remove moisture in Medical air production. All makes and models employ activated alumina and/or molecular sieve, both of which attract moisture and carbon dioxide.
Through our aerALin™ quality control system, we discovered that current industry standard methods for operating dessicant dryers, cause them to capture and release carbon dioxide into the process air stream. The result is chronically off-spec Medical air with levels of carbon dioxide exceeding the USP limit of 500ppm.
When compared with dewpoint, we noticed inconsistent waveform amplitudes for CO2, principally due to varying outdoor air quality and dryer operational settings.
P3C provides unparalleled Medical air quality to protect vulnerable lives
- Real time monitoring & traceability of USP parameters
- Automatic off-spec prevention
- Trend based remediation
- Patient Protect Purge Control
Key features
-
User-friendly touchscreen operator interface
-
Connectivity for alarm event logging and trend graphs/information
-
Available for purchase, rent-to-own, or on a monthly facility fee basis
-
Made in Canada and supported by our national service network
Quality control essential to ensuring patient safety
At most facilities, you will find it is made on-site for economic reasons. The quality of Medical air produced is directly linked to the quality of the raw material, outdoor air.
To help ensure your patients are administered precise Medical air USP, we developed aerALin™ — our parametric batch release quality control system. It validates your on-site Medical air USP to meet good manufacturing practices and reflect the production quality control experience from our plants.
Related resources
-
P3C Dryer - Brochure
Download the document PDF (1.87 MB) -
Medical air USP - Brochure
Download the document PDF (973.9 KB) -
Master Medical Gas System Specification
Download the document PDF (540.41 KB) -
Air Liquide Healthcare / BUSCH - Medical Air - Brochure
Download the document PDF (2.75 MB)
Do you need pricing or more information?
Our team will listen to your needs and answer your questions, to help identify the right equipment and services for your facility.
Write us
Talk to us
Visit us
6990 Creditview Road, Unit 6
Mississauga, ON L5N 8R9
Why choose us
-
Full-scope medical gases provider
We are involved from production to patient therapy, and know medical gases intimately -
Reliability
You can depend on us to provide you with the right equipment and services to meet your facility's needs -
Innovation
Innovation is our vision: we prioritize quality of care through patient-centric products and services -
Safety
Ensuring the safety of your patients and staff is our top priority - our emphasis is on safety, compliance and risk management -
Experienced
We are owned by Air Liquide Group, the largest supplier of industrial, medical & specialty gases, found on every continent in the world -
Coast-to-coast service network
Our fill plants are widespread to shorten response-time and ensure supply chain reliability -
Accreditation and regulation
We help you ensure your facility's medical equipment is serviced and fully compliant with Canadian standards -
Customized portfolio
We supply a complete range of high-quality specialty gases, including mixtures used in specific applications to suit your needs